BREAKDOWN IN SOLID DIELECTRICS
Solid insulating materials are used almost in all electrical equipments, be it an electric heater or a 500 MW generator or a circuit breaker, solid insulation forms an integral part of all electrical equipments especially when the operating voltages are high. The solid insulation not only provides insulation to the live parts of the equipment from the grounded structures, it sometimes provides mechanical support to the equipment. In general, of course, a suitable combination of solid, liquid and gaseous insulations is used.
The processes responsible for the breakdown of gaseous dielectrics are governed by the rapid growth of current due to emission of electrons from the cathode, ionization of the gas particles and fast development of avalanche process. When breakdown occurs the gases regain their dielectric strength very fast, the liquids regain partially and solid dielectrics lose their strength completely.
The breakdown of solid dielectrics not only depends upon the magnitude of voltage applied but also it is a function of time for which the voltage is applied. Roughly speaking, the product of the breakdown voltage and the log of the time required for breakdown is almost a constant i.e,
Vb = 1n tb = constant
The characteristics is shown in Fig. 2.11
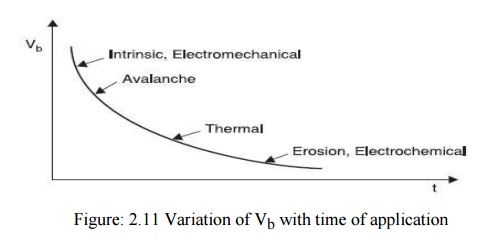
The dielectric strength of solid materials is affected by many factors viz. ambient temperature, humidity, duration of test, impurities or structural defects whether a.c., d.c. or impulse voltages are being used, pressure applied to these electrodes etc. The mechanism of breakdown in solids is again less understood. However, as is said earlier the time of application plays an important role in breakdown process, for discussion purposes, it is convenient to divide the time scale of voltage application into regions in which different mechanisms operate. The various mechanisms are:
· Intrinisic Breakdown
· Electromechanical Breakdown
· Breakdown Due to Treeing and Tracking
· Thermal Breakdown
· Electrochemical Breakdown
1. Intrinsic breakdown in solids
If the dielectric material is pure and homogeneous, the temperature and environmental conditions suitably controlled and if the voltage is applied for a very short time of the order of 10–8 second, the dielectric strength of the specimen increases rapidly to an upper limit known as intrinsic dielectric strength. The intrinsic strength, therefore, depends mainly upon the structural design of the material i.e., the material itself and is affected by the ambient temperature as the structure itself might change slightly by temperature condition.
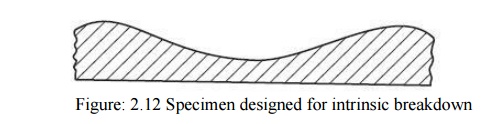
In order to obtain the intrinsic dielectric strength of a material, the samples are so prepared that there is high stress in the centre of the specimen and much low stress at the corners
as shown in Fig. 2.12. The intrinsic breakdown is obtained in times of the order of 10–8 sec. and, therefore, has been considered to be electronic in nature.
The stresses required are of the order of one million volt/cm. The intrinsic strength is generally assumed to have been reached when electrons in the valance band gain sufficient energy from the electric field to cross the forbidden energy band to the conduction band. In pure and homogenous materials, the valence and the conduction bands are separated by a large energy gap at room temperature, no electron can jump from valance band to the conduction band.
The conductivity of pure dielectrics at room temperature is, therefore, zero. However, in practice, no insulating material is pure and, therefore, has some impurities and/or imperfections in their structural designs. The impurity atoms may act as traps for free electrons in energy levels that lie just below the conduction band is small. An amorphous crystal will, therefore, always have some free electrons in the conduction band. At room temperature some of the trapped electrons will be excited thermally into the conduction band as the energy gap between the trapping band and the conduction band is small.
An amorphous crystal will, therefore, always have some free electrons in the conduction band. As an electric field is applied, the electrons gain energy and due to collisions between them the energy is shared by all electrons. In an amorphous dielectric the energy gained by electrons from the electric field is much more than they can transfer it to the lattice. Therefore, the temperature of electrons will exceed the lattice temperature and this will result into increase in the number of trapped electrons reaching the conduction band and finally leading to complete breakdown. When an electrode embedded in a solid specimen is subjected to a uniform electric field, breakdown may occur.
An electron entering the conduction band of the dielectric at the cathode will move towards the anode under the effect of the electric field. During its movement, it gains energy and on collision it loses a part of the energy. If the mean free path is long, the energy gained due to motion is more than lost during collision. The process continues and finally may lead to formation of an electron avalanche similar to gases and will lead finally to breakdown if the avalanche exceeds a certain critical size.

