Liquid dielectrics are used for filling transformers, circuit breakers and as impregnates in high voltage cables and capacitors. For transformer, the liquid dielectric is used both for providing insulation between the live parts of the transformer and the grounded parts besides carrying out the heat from the transformer to the atmosphere thus providing cooling effect. For circuit breaker, again besides providing insulation between the live parts and the grounded parts, the liquid dielectric is used to quench the arc developed between the breaker contacts. The liquid dielectrics mostly used are petroleum oils. Other oils used are synthetic hydrocarbons and halogenated hydrocarbons and for very high temperature applications sillicone oils and fluorinated hyrocarbons are also used.
The three most important properties of liquid dielectric are (i) The dielectric strength (ii) The dielectric constant and (iii) The electrical conductivity. Other important properties are viscosity, thermal stability, specific gravity, flash point etc. The most important factors which affect the dielectric strength of oil are the, presence of fine water droplets and the fibrous impurities. The presence of even 0.01% water in oil brings down the dielectric strength to 20% of the dry oil value and the presence of fibrous impurities brings down the dielectric strength much sharply. Therefore, whenever these oils are used for providing electrical insulation, these should be free from moisture, products of oxidation and other contaminants.
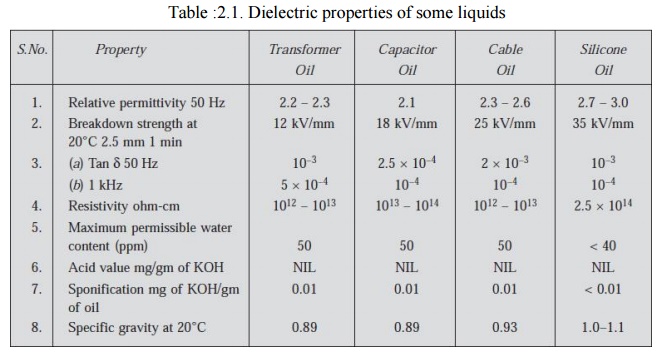
The main consideration in the selection of a liquid dielectric is its chemical stability. The other considerations are the cost, the saving in space, susceptibility to environmental influences etc. The use of liquid dielectric has brought down the size of equipment tremendously. In fact, it is practically impossible to construct a 765 kV transformer with air as the insulating medium. Table 2.1. Shows the properties of some dielectrics commonly used in electrical equipments.
Liquids which are chemically pure, structurally simple and do not contain any impurity even in traces of 1 in 109, are known as pure liquids. In contrast, commercial liquids used as insulating liquids are chemically impure and contain mixtures of complex organic molecules. In fact their behaviour is quite erratic. No two samples of oil taken out from the same container will behave identically. The theory of liquid insulation breakdown is less understood as of today as compared to the gas or even solids. Many aspects of liquid breakdown have been investigated over the last decades but no general theory has been evolved so far to explain the breakdown in liquids. Investigations carried out so far, however, can be classified into two schools of thought.
The first one tries to explain the breakdown in liquids on a model which is an extension of gaseous breakdown, based on the avalanche ionization of the atoms caused by electon collisiron in the applied field. The electrons are assumed to be ejected from the cathode into the liquid by either a field emission or by the field enhanced thermionic effect (Shottky’s effect).
This breakdown mechanism explains breakdown only of highly pure liquid and does not apply to explain the breakdown mechanism in commercially available liquids.
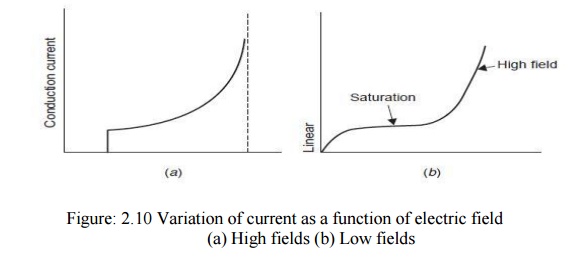
It has been observed that conduction in pure liquids at low electric field (1 kV/cm) is largely ionic due to dissociation of impurities and increases linearily with the field strength. At moderately high fields the conduction saturates but at high field (electric), 100 kV/cm the conduction increases more rapidly and thus breakdown takes place. Fig. 2.10 (a) shows the variation of current as a function of electric field for hexane. This is the condition nearer to breakdown. However, if the figure is redrawn starting with low fields, a current-electric field characteristic as shown in Fig. 2.10 (b) will be obtained.
The second school of thought recognises that the presence of foreign particles in liquid insulations has a marked effect on the dielectric strength of liquid dielectrics. It has been suggested that the suspended particles are polarizable and are of higher permittivity than the liquid. These particles experience an electrical force directed towards the place of maximum stress. With uniform field electrodes the movement of particles is presumed to be initiated by surface irregularities on the electrodes, which give rise to local field gradients. The particles thus get accumulated and tend to form a bridge across the gap which leads finally to initiation of breakdown. The impurities could also be in the form of gaseous bubbles which obviously have lower dielectric strength than the liquid itself and hence on breakdown of bubble the total breakdown of liquid may be triggered.
1. Electronic Breakdown
Once an electron is injected into the liquid, it gains energy from the electric field applied between the electrodes. It is presumed that some electrons will gain more energy due to field than they would lose during collision. These electrons are accelerated under the electric field and would gain sufficient energy to knock out an electron and thus initiate the process of avalanche. The threshold condition for the beginning of avalanche is achieved when the energy gained by the electron equals the energy lost during ionization (electron emission) and is given by
e λ E = Chv
where λ is the mean free path, hv is the energy of ionization and C is a constant. Table
2.2 gives typical values of dielectric strengths of some of the highly purified liquids.
The electronic theory whereas predicts the relative values of dielectric strength satisfactorily, the formative time lags observed are much longer as compared to the ones predicted by the electronic theory.
Table: 2.2. Dielectric strengths of pure liquids