Fuel cell with Schematic diagram
A Fuel cell is an electrochemical device in which the chemical energy of a conventional fuel is converted directly and efficiently into low voltage, direct-current electrical energy. One of the chief advantages of such a device is that because the conversion, atleast in theory, can be carried out isothermally, the Carnot limitation on efficiency does not apply. A fuel cell is often described as primary battery in which the fuel and oxidizer are stores external to the battery and fed to it as needed.
Fig. shows a schematic diagram of a fuel cell. The fuel gas diffuses through the anode and is oxidized, thus releasing electrons to the external circuit; the oxidizer diffuses through the cathode and is reduced by the electrons that have come from the anode by way of the external circuit.
The fuel cell is a device that keeps the fuel molecules from mixing with the oxidizer molecules, permitting, however, the transfer of electrons by a metallic path that may contain a load.
Of the available fuels, hydrogen has so far given the most promising results, although cells consuming coal, oil or natural gas would be economically much more useful for large scale applications.
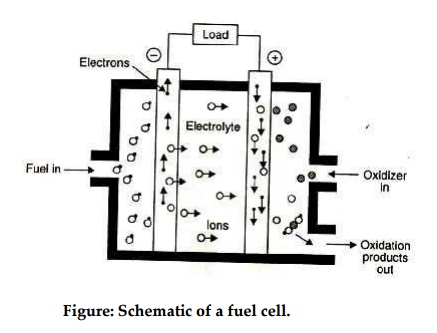
Figure: Schematic of a fuel cell.
Some of the possible reactions are :
Hydrogen/oxygen 1.23 V : 2H2 + O2 -> 2 H2O
Hydrazine 1.56 V N2H4 + O2 -> 2H2O + N2
Carbon (coal) 1.02 V C + O2 -> CO2
Methane 1.05 V CH4 + 2O2 -> CO2 + 2H2O
Hydrogen-oxygen cell :
The hydrogen-oxygen devices shown in figure is typical of fuel cells. It has three chambers separated by two porous electrodes, the anode and the cathode. The middle chamber between the electrodes is filled with a strong solution of potassium hydroxide. The surfaces of the electrodes are chemically treated to repel the electrolyte, so that there is minimum leakage of potassium hydroxide into the outer chambers. The gases diffuse through the electrodes, undergoing reactions are show below:
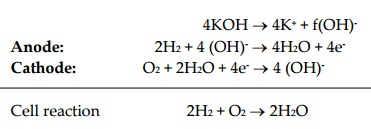
4KOH ® 4K+ + f(OH)-
Anode: 2H2 + 4 (OH)- ® 4H2O + 4e-
Cathode: O2 + 2H2O + 4e- ® 4 (OH)-
Cell reaction 2H2 + O2 ® 2H2O
The water formed is drawn off from the side. The electrolyte provides the (OH)- ions needed for the reaction, and remains unchanged at the end, since these ions are regenerated. The electrons liberated at the anode find their way to the cathode through the external circuit. This transfer is equivalent to the flow of a current from the cathode to the anode.
Such cells when properly designed and operated, have an open circuit voltage of about 1.1 volt. Unfortunately, their life is limited since the water formed continuously dilutes the electrolyte. Fuel efficiencies as high as 60%-70% may be obtained.
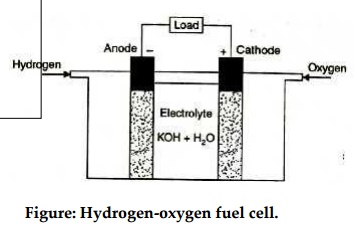
Figure: Hydrogen-oxygen fuel cell.

