IONIZATION AND DECAY PROCESSES
At normal temperature and pressure gases are excellent insulators. The conduction in air at low field is in the region 10-16 – 10-17 A/cm2. These current results from cosmic radiations and radioactive substances present in earth and the atmosphere. At higher fields charged particles may gain sufficient energy between collisions to cause ionization on impact with neutral molecules.
It was shown in the previous section that electrons on average lose little energy in elastic collisions and readily build up their kinetic energy which may be supplied by an external source, e.g. an applied field. On the other hand, during inelastic collisions a large fraction of their kinetic energy is transferred into potential energy, causing, for example, ionization of the struck molecule. Ionization by electron impact is for higher field strength the most important process leading to breakdown of gases. The effectiveness of ionization by electron impact depends upon the energy that an electron can gain along the mean free path in the direction of the field.
This simple model is not applicable for quantitative calculations, because ionization by collision, as are all other processes in gas discharges, is a probability phenomenon, and is generally expressed in terms of cross-section for ionization defined as the product Piσ = σi where Pi is the probability of ionization on impact and σ is the molecular or atomic cross-sectional area for interception defined earlier. The cross-section &i is measured using monoenergetic electron
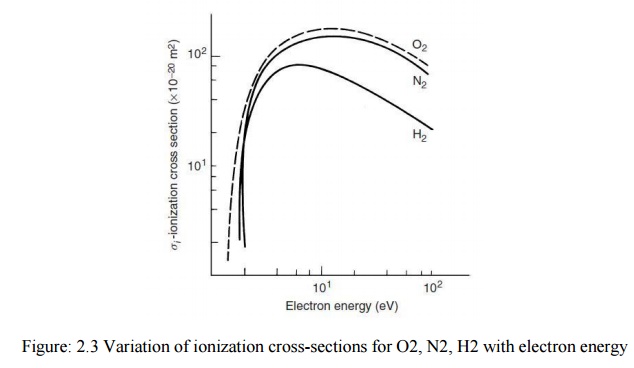
beams of different energy. The variation of ionization cross-sections for H2, O2, and N2 with electron energy.
It is seen that the cross-section is strongly dependent upon the electron energy. At energies below ionization potential the collision may lead to excitation of the struck atom or molecule which on collision with another slow moving electron may become ionized. This process becomes significant only when densities of electrons are high. Very fast moving electrons may pass near an atom without ejecting an electron from it. For every gas there exists an optimum electron energy range which gives a maximum ionization probability.

